A Guide to Cleanroom Certifications and Regulations

Cleanrooms are specialized environments that are designed to control the level of contaminants in the air. They are commonly used in industries such as pharmaceuticals, semiconductors, and medical device manufacturing. In North America and Europe, cleanrooms are subject to a variety of certifications and regulations to ensure that they meet industry standards for cleanliness and safety.
In North America, the Federal Drug Administration (FDA) is responsible for overseeing the regulations for cleanrooms used in the pharmaceutical industry. The FDA’s regulations are outlined in the Current Good Manufacturing Practices (cGMP) guidelines, which set standards for the design, construction, and operation of cleanrooms. These regulations cover a range of issues, including air filtration, temperature and humidity control, and personnel practices.
Similarly, in Canada, Health Canada oversees the regulations for cleanrooms used in the pharmaceutical industry. These regulations are outlined in the Good Manufacturing Practices (GMP) guidelines, which are similar to the FDA’s cGMP guidelines. The GMP guidelines cover the same range of issues as the FDA’s regulations and aim to ensure that cleanrooms used in the pharmaceutical industry meet standards for cleanliness and safety.
In Europe, the cleanroom regulations are set by the European Union (EU). The EU’s regulations are outlined in the European Union GMP guidelines, which are similar to the FDA’s cGMP and Health Canada’s GMP guidelines. These regulations cover a range of issues, including air filtration, temperature and humidity control, and personnel practices and aim to ensure that cleanrooms used in the pharmaceutical industry meet standards for cleanliness and safety.
One of the most widely recognized cleanroom certifications in North America and Europe is ISO 14644-1, which is issued by the International Organization for Standardization (ISO). This certification sets standards for the design, construction, and operation of cleanrooms, and is recognized by the FDA, Health Canada, and the EU.
It’s worth noting that the regulations and guidelines for cleanroom design, construction and operation may vary depending on the specific industry and the use of the cleanroom, it’s important to consult with experts and comply with the relevant regulations and guidelines before building a cleanroom.

North American GMP and EU GMP Certifications
The Current Good Manufacturing Practices (cGMP) guidelines set by the Federal Drug Administration (FDA) in North America and the European Union GMP (EU GMP) guidelines set by the European Union (EU) have some similarities, but also have some differences.
Both the cGMP and EU GMP guidelines are in place to ensure that cleanrooms used in the pharmaceutical industry meet standards for cleanliness and safety. Both set standards for the design, construction, and operation of cleanrooms including air filtration, temperature and humidity control, and personnel practices.
One main difference between the two sets of guidelines is that the EU GMP guidelines are more comprehensive and detailed than the cGMP guidelines, with more specific requirements for certain areas such as the qualification of equipment, the calibration of instruments and the maintenance of records.
Another difference is that the EU GMP guidelines have more stringent requirements for the sanitation and hygiene of facilities, equipment, and personnel, as well as a stronger emphasis on risk management.
Additionally, while the FDA focuses on compliance with the regulations and guidelines, the EU has a more risk-based approach which means that the EU’s GMP guidelines place more emphasis on the manufacturer’s own evaluation of the risks and hazards associated with their products and processes, and the implementation of appropriate controls to manage those risks.
It’s worth mentioning that both sets of guidelines are subject to change and updates, so it’s important to keep abreast of any changes and updates to the regulations to ensure compliance during certifications.
Cleanroom Certifications
Certifying a cleanroom involves a process of testing and evaluating the cleanroom to ensure that it meets the standards set by the relevant regulatory bodies and industry standards. The specific steps involved in certifying a cleanroom can vary depending on the industry and the specific regulations or guidelines that must be followed, but generally, the process includes the following steps:
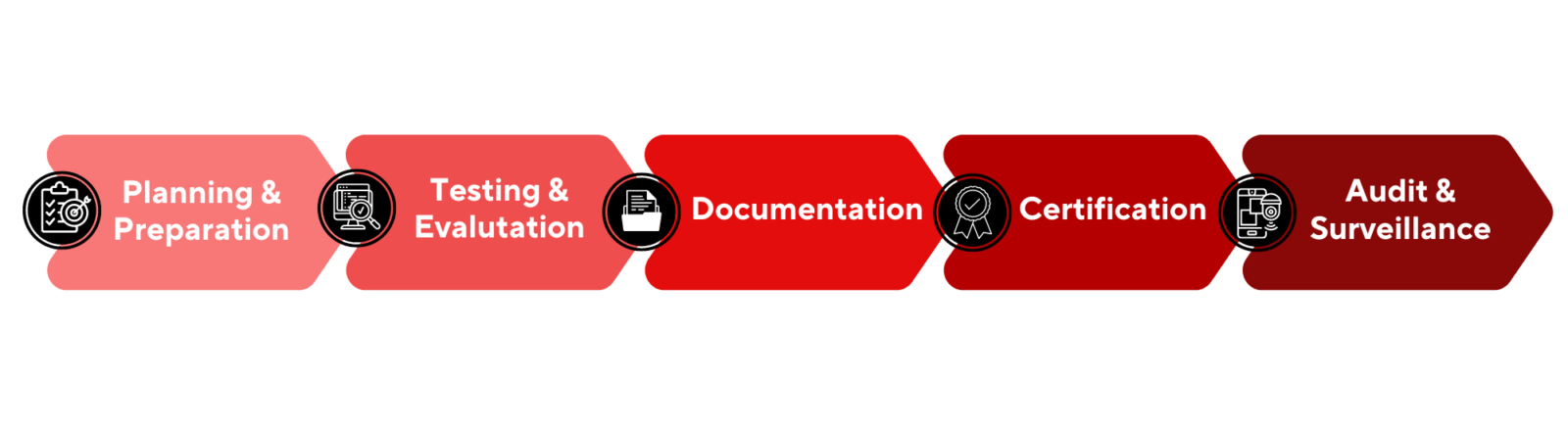
- Planning and Preparation: The first step in certifying a cleanroom is to plan and prepare for the certification process. This includes identifying the specific regulations or guidelines that must be followed and the standards that must be met, as well as determining the testing and evaluation methods that will be used.
- Testing and Evaluation: The next step is to test and evaluate the cleanroom to ensure that it meets the relevant standards. This can include testing the air quality, temperature, and humidity levels, as well as evaluating the materials, equipment, and personnel practices used in the cleanroom.
- Documentation: After testing and evaluation, a report is generated that documents the results of the testing and evaluation process. This report should include the test results, as well as any observations or recommendations made during the evaluation.
- Certification: Once the report is generated, the cleanroom will be certified if it meets the standards set by the regulatory bodies and industry standards. A certificate is issued to the facility that includes the results of the testing and evaluation process, as well as the specific standards that were met.
- Audit and Surveillance: After certification, regular audits and surveillance are done to ensure that the cleanroom continues to meet the standards.
It’s worth noting that certifications can be time-consuming and costly, but it is necessary to ensure that the cleanroom meets the relevant standards and regulations, as well as to demonstrate compliance to customers, regulatory agencies, and other stakeholders.
Required Testing for Certification of a Cleanroom
The specific testing required to certify a cleanroom can vary depending on the industry, the specific regulations or guidelines that must be followed, and the type of cleanroom. However, here are some common tests that are typically performed during the certification process:
- Particle counting: This test measures the number of particles in the air and is used to determine the cleanliness level of the room.
- Airflow measurement: This test measures the airflow patterns within the room and is used to ensure that the room is being properly ventilated.
- Temperature and humidity measurement: This test measures the temperature and humidity within the room and is used to ensure that the room is being maintained at the appropriate temperature and humidity levels.
- Microbial air and surface test: This test measures the levels of microorganisms in the air and on surfaces and is used to ensure that the cleanroom is free of harmful microorganisms.
- Air change rate test: This test measures the number of air changes per hour in the cleanroom and is used to ensure that the room is being properly ventilated.
Don’t delay in taking this important step to maintain the highest levels of cleanliness and safety in your cleanroom facility. Reach out us at info@achengineering.com today and embark on your journeytowards cleanroom certification!
GET IN TOUCH
Complete the form below to get in touch with our team.
